Therapeutic potential and liability of the Mitragyna speciosa (kratom) alkaloids
Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine

Abstract
Kratom, derived from the plant Mitragyna speciosa, is receiving increased attention as an alternative to traditional opi- ates and as a replacement therapy for opiate dependence. Mitragynine (MG) and 7-hydroxymitragynine (7-HMG) are major psychoactive constituents of kratom. While MG and 7-HMG share behavioral and analgesic properties with mor- phine, their reinforcing effects have not been examined to date. 7-HMG, but not MG, substituted for morphine self- administration in a dose-dependent manner in the rat self-administration paradigm. Following the substitution proce- dure, re-assessment of morphine self-administration revealed a significant increase following 7-HMG and a significant decrease following MG substitution. In a separate cohort, 7-HMG, but not MG, engendered and maintained intrave- nous self-administration in a dose-dependent manner. The effects of pretreatment with nalxonaxine (NLXZ), a μ1 opi- ate receptor antagonist, and naltrindole (NTI), a δ opiate receptor antagonist, on 7-HMG and morphine self- administration were also examined. Both NLXZ and NTI reduced 7-HMG self-administration, whereas only NLXZ de- creased morphine intake. The present results are the first to demonstrate that 7-HMG is readily self-administered, and the reinforcing effects of 7-HMG are mediated in part by μ and δ opiate receptors. In addition, prior exposure to 7-HMG increased subsequent morphine intake whereas prior exposure to MG decreased morphine intake. The present findings indicate that MG does not have abuse potential and reduces morphine intake, desired characteristics of candidate phar- macotherapies for opiate addiction and withdrawal, whereas 7-HMG should be considered a kratom constituent with high abuse potential that may also increase the intake of other opiates.
Introduction
The increasing use of kratom (Mitragyna speciosa korth; aka thang, kakuam, thom, ketum, biak biak), a plant indig- enous to Southeast Asia, has emerged as a public health concern in the US. Kratom has been used traditionally to combat fatigue and increase work productivity amongst farm populations in Southeast Asia. Kratom leaves are chewed or made into an extract and brewed (Hassan et al. 2013; Warner, Kaufman, & Grundmann 2016), and consumption is reported to produce stimulation (at low doses) and opiate-like effects (at higher doses) including analgesia, antitussive, antidiarrheal, and anti-inflammatory effects.
Historically, kratom has also been used to reduce the intensity and duration of opiate withdrawal symp- toms (Boyer et al. 2008; Ward et al. 2011; Cinosi et al. 2015; Warner et al. 2016); however, studies assessing the clinical efficacy of kratom are limited. In Southeast Asia, regular kratom use has been associated with phys- ical dependence and withdrawal symptoms (Suwanlert 1975; Saingam et al. 2013; Singh, Muller, & Vicknasingam 2014), effects attributed in large part to mitragynine (MG). In the United States, there is growing concern regarding the safe use of kratom based on reports of addiction (Sheleg & Collins 2011; Galbis-Reig 2016) and toxicity and fatalities associated with use (McWhirter & Morris 2010; Neerman, Frost, & Deking 2013; Singh, Narayanan, & Vicknasingam 2016; Drago et al. 2017; Fluyau & Revadigar 2017).
Various strains of kratom are widely available over the internet as well as in various locations throughout the country. Forecasting models indicate kratom use will con- tinue to increase in the United States (Stogner 2015). Currently, kratom consumption remains legal in the ma- jority of states in the US. FDA’s associate commissioner for regulatory affairs has stated that the FDA has ‘identified Kratom as a botanical substance that poses a risk to pub- lic health and has the potential for abuse’ (Food and Drug Administration, 2016). Concerns about Kratom have led the FDA to issue an import alert and public health advi- sory and the DEA to include kratom on the list of Drugs and Chemicals of Concern.
Related: Kratom Legality Map and information. States, Cities and Counties where Kratom products are prohibited
Kratom leaves contain more than 25 identified alkaloids (Hassan et al. 2013). Mitragynine (MG) and 7- hydroxymitrgynine (7-HMG), the main psychoactive alkaloids of kratom, constitute approximately 60 and 2 percent of the plant’s alkaloids, respectively (Prozialeck, Jivan, & Andurkar 2012). MG and 7-HMG are partial agonists at the μ opiate receptor and weak antagonists at δ and κ opiate receptors (Kruegel et al. 2016; Varadi et al. 2016), with 7-HMG exhibiting approximately 5-fold greater affinity at the μ opiate receptor compared to MG. Assays of opiate receptor-mediated G-protein function re- veal similar potencies for MG and 7-HMG (Kruegel et al. 2016; Varadi et al. 2016). While both compounds exhibit naloxone-sensitive antinociceptive activity, 7-HMG exhibits 40-fold greater potency than MG and 10-fold greater potency than morphine in these assays (Takayama et al. 2002; Matsumoto et al. 2004). Repeated administration of 7-HMG produces tolerance to the compound’s analgesic effects as well as cross-tolerance to morphine’s antinociceptive action (Matsumoto et al. 2005). Chronic consumption of kratom as well as repeated administration of 7-HMG induces physical dependence as determined by naloxone-precipitated withdrawal (Matsumoto et al. 2005).
Few studies have examined the abuse/addiction po- tential of MG and 7-HMG. The absence of controlled stud- ies in humans creates space for basic science studies to provide critical information to help guide use and policy decisions. The abuse liability of compounds is generally assessed in animal models using drug discrimination, place conditioning and/or self-administration paradigms —all of which address different aspects of abuse. Studies in animal models have provided considerable insight into the behavioral effects of MG and 7-HMG as well as the po- tential neurobiological mechanisms underlying those ef- fects. Acute administration of MG increases locomotor activity, induces anxiolytic effects and induces condi- tioned place preference (Yusoff et al. 2016), while re- peated MG administration induces locomotor sensitization but impairs performance on a variety of cognitive tasks (Yusoff et al. 2016; Ismail et al. 2017). Moreover, MG and 7-HMG fully generalize to the discrim- inative stimulus effects of morphine, suggesting the potential for abuse (Harun et al., 2015).
The reinforcing effects of drugs are an important indi- cator of abuse liability which is typically evaluated using drug self-administration procedures. Inherent in the op- erational definition of reinforcement is the contingency between behavior and drug administration, an important differentiation between self-administration and place conditioning and drug discrimination. Drug self- administration is widely accepted as the gold standard of measurements for abuse liability (Katz 1989; Hemby 1999; Lynch & Hemby 2011). In spite of the concern of the potential abuse liability of MG and 7-HMG in humans, no published studies to date have examined the ability of these compounds to maintain self- administration in experimental subjects. To that end, we assessed that ability of MG and 7- HMG to substitute for morphine self-administration and to engender and main- tain self-administration in drug naïve animals. Addition- ally, the contribution of μ and δ opiate receptors on the reinforcing effects of 7-HMG was examined. Results of these studies provide an objective assessment of the abuse potential of MG and 7-HMG in a rodent model that reca- pitulates key features of human drug taking.
MATERIALS AND METHODS
Subjects
Male Fischer 344 rats (100–130 days; Charles River, Wil- mington, MA) were housed in a temperature-controlled vivarium on a 12-hour reversed light/dark cycle (lights on at 6:00 PM). Rats were group-housed two per cage with water available ad libitum. Food was restricted such that rats were maintained at 90 percent of their free feed- ing weight throughout the experiment. Experimental ses- sions were conducted during the dark phase of the light/dark cycle. All procedures were performed in accor- dance with the High Point University Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996.
Chemicals
Mitragynine was isolated according to published proce- dures (Ponglux et al. 1994). 7-HMG was synthesized from mitragynine by McCurdy’s research group, Department of BioMolecular Sciences, University of Mississippi, as previ- ously published (Ponglux et al. 1994; Takayama et al. 2002). Mitragynine and 7-HMG were analyzed by 1H NMR, 13C NMR, elemental analysis, HPLC and HR-MS, and were found to be >99 percent pure. Morphine SO4 was purchased from Gallipot, Inc. (St. Paul, MN), penicillin G procaine from Butler Company (Columbus, OH), propofol, ketamine HCl (Ketaset), xylazine (Xylamed) from Patterson Veterinary Supply, Inc. (Greely, CO), naltrindole and naloxonazine from Tocris Bioscience/Biotechne (Minneapolis, MN). Drugs were dissolved in heparinized saline. Morphine, MG and 7-HMG were infused in a volume of 200 μl.
Behavioral apparatus and training
Operant apparatus
Experiments were conducted in operant conditioning chambers (ENV-008CT; Med Associates, St. Albans, VT) enclosed in sound-attenuating cubicles (ENV-018; Med Associates). The front panel of the operant chambers contained a response lever (4 cm above the floor and 3 cm from the side wall), a cue light (3 cm above the lever) and a food chute centered on the front wall (2 cm above the floor) that was connected to a food pellet dispenser (ENV-023; Med Associates) located behind the front wall and a tone generator to mask extraneous noise. A syringe pump (PHM-100; Med Associates) holding a 20-ml syringe delivered infusions. A counter- balanced arm containing the single channel liquid swivel was located 8–8.5 cm above the chamber and attached to the outside of the front panel. An IBM compatible computer was used for session programming and data collection (Med Associates Inc., East Fairfield, VT).
Lever training
Subjects were transferred to the operant chambers for daily experimental sessions, and responding was engen- dered and maintained by delivery of food pellets (45-mg pellets; Noyes, Lancaster, NH) under an FR 1 schedule of reinforcement that was gradually increased to FR3 (every third response produced a food pellet). The lever light was illuminated when the schedule was in effect. Completion of the response requirement extinguished lights, delivered food and was followed by a 20-second timeout (TO) period during which all lights were extinguished, and responses had no scheduled consequences. After the TO, the lights were illuminated, and the FR schedule was again in effect. Sessions lasted 20 minutes or until 30 food pellets were delivered. Responding was considered stable when there was less than 10 percent variation in the number of rein- forcers for three consecutive sessions.
Intravenous jugular surgery
After operant responding was acquired and maintained by food, subjects surgically implanted with an intravenous jugular catheter. Venous catheters were inserted into the right jugular vein following administra- tion of ketamine (90 mg/kg; IP) and xylazine (5 mg/kg; IP) for anesthesia as described previously (Pattison et al. 2012; Pattison et al. 2014; McIntosh et al. 2015). Cathe- ters were anchored to muscle near the point of entry into the vein. The distal end of the catheter was guided subcu- taneously to exit above the scapulae through a Teflon shoulder harness. The harness provided a point of attach- ment for a spring leash connected to a single-channel fluid swivel at the opposing end. The catheter was threaded through the leash and attached to the swivel. The other end of the swivel was connected to a syringe (for saline and drug delivery) mounted on a syringe pump. Rats were administered penicillin G procaine (75 000 units in 0.25 ml, i.m.) and allowed a minimum of 5 days to recover before self-administration studies were initiated. Hourly infusions of heparinized saline were administered through the catheter to maintain functional catheters. The health of the rats was moni- tored daily by the experimenters and weekly by institu- tional veterinarians per the guidelines issued by the Institutional Animal Care and Use Committee and the National Institutes of Health. Infusions of propofol (6 mg/kg; i.v.) were manually administered as needed to assess catheter patency.
Rats were transferred to the operant chambers for daily two-hour self-administration sessions. Before each session, the swivel and catheter were flushed with 500 μl of heparinized saline before connecting the cathe- ter to the syringe via a 20 ga luer hub and 28 ga male connector. The start of each session was indicated by the illumination of the house light, stimulus light above the lever and the extension of the lever. Completion of the response requirement was followed by a 20-second time out (FR3:TO 20 seconds) during which time the subject received a 200-μl intravenous infusion over the first 6 seconds, retraction of the lever, extinguishing of le- ver light, generation of a tone and illumination of the house light. At the end of the TO, the lever was extended, lever light illuminated, tone silenced and the house light extinguished (Hemby, Smith, & Dworkin 1996; Hemby et al. 1999; McIntosh et al. 2015).
Experiment 1: Substitution for morphine self-administration
Self-administration was engendered using 100 μg/inf of morphine sulfate. When responding was stable (two con- secutive sessions in which the number of reinforcers did not vary by more than 20 percent), the dose was changed to 50 μg/inf. Following stable responding at this dose, saline was substituted for morphine until responding stabilized. Following the conclusion of extinc- tion testing, rats were assigned to one of two groups to
receive MG (n = 9; 25, 50, 100 and 150 μg/inf) or 7-HMG (n = 8; 2.5, 5, 10 and 20 μg/inf)—doses were randomized. The dose range for MG self-administration was based on the finding that equivalent doses of mor- phine and MG induced place conditioning in rats (Yusoff et al. 2016). The dose range for 7-HMG was based on the finding that 7-HMG substitutes for morphine in the drug discrimination procedure at a dose five-fold lower than the training dose of morphine. Once responding stabilized for a particular dose, the next dose was made available the following session until all doses mentioned above were assessed. After all doses had been assessed for a sub- ject within a group, the rat was allowed to self-administer morphine (50 and 100 μg/inf) to determine the effect of prior drug history on subsequent morphine intake.
Experiment 2: Acquisition of self-administration
Whereas substitution procedures may be more sensitive for determining the reinforcing effects of a compound, acquisition procedures assess only the reinforcing effects of a single compound, without the potential confound of prior drug associations. The ability of MG and 7-HMG to engender and maintain responding without prior drug history was determined in a separate cohort of rats. Rats were assigned to one of three groups to self-administer MG (n = 8; 100 μg/infusion), 7-HMG (n = 8; 10 μg/infusion) or morphine (n = 6; 100 μg/infusion). Fol- lowing stable responding at the initial dose, rats in the MG group were given access to 25 and 50 μg/infusion MG, 7-HMG group was allowed to self-administer 5.0 and 2.5 μg/infusion 7-HMG, while rats in the morphine group were allowed to self-administer 50 μg/infusion morphine. The presentation of doses for MG and 7-HMG was randomized following the initial dose.
Experiment 3: Selective opiate receptor antagonism of morphine and 7-HMG self-administration
To determine the contribution of μ and δ opiate receptors on the reinforcing effects of 7-HMG, rats that had previ- ously acquired morphine and 7-MHG self-administration were administered naloxonazine (NLXZ), a selective μ1 receptor antagonist or the δ receptor antagonist naltrindole (NTI). NLXZ (5 and 15 mg/kg, i.p.) and NTI (0.5 and 5 mg/kg, i.p.) were administered 30 minutes before the session. The effects of saline pretreatment and saline extinction on responding were also assessed.
Data analysis
Experiment 1
Morphine self-administration as well as MG and 7-HMG substitution was analyzed using a one-factor ANOVA (Dose). Morphine self-administration before and after MG and 7-HMG substitution was analyzed using a two- way ANOVA (Dose × Pre/Post). Analysis of three days fol- lowing MG and 7-HMG substitution was conducted using a two-way repeated measures ANOVA (Dose × Pre/Post) with Sessions as the repeated measure.
Experiment 2
Acquisition of self-administration was analyzed using one-factor ANOVA (Dose) ANOVA and number of infu- sions as the dependent measure.
Experiment 3
Com- parisons between baseline intake and intake following saline pretreatment were conducted using two-tailed paired t-test for both the morphine and 7-HMG groups. The effects of NLXZ and NTI on morphine and 7-HMG were analyzed independently using a one-factor ANOVA (Antagonist Dose).
Analysis of the session in which the antagonists or saline were administered, and the follow- ing session was conducted using a two-way repeated measures ANOVA (Dose × Day) with Sessions as the repeated measure. The number of infusions was the dependent variable for analyses. Where appropriate, post hoc analyses were conducted using Tukey’s test.
RESULTS
Substitution and reintroduction of morphine
Responding was engendered and maintained by mor- phine at the doses tested [F(2,17) = 24.4, P < 0.0001]. Morphine self-administration was dose-dependent with both morphine doses significantly greater than saline and 50 μg greater than 100 μg/infusion (P < 0.05) (Fig. 1a). MG was not reliably self-administered when substituted for morphine [F(4,38) = 0.51, P = 0.73]. MG intake was not significantly different from vehicle, suggesting that MG does not function as a reinforcer at the doses tested (Fig. 1b). In contrast, 7-HMG substituted for morphine with intake dependent on the dose available [F(4,32) = 6.3, P = 0.0009]. The number of infusions obtained for 5 and 10 μg/inf were significantly greater than vehicle (P < 0.05), confirming that these doses of 7-HMG functioned as reinforcing stimuli. 7-HMG resulted in an inverted ‘U’-shaped dose-effect function with maxi- mal intake observed at 5 and 10 μg/inf (Fig. 1c).
Following completion of the substitution protocol, morphine self-administration was re-assessed. Morphine intake was significantly altered by both MG [F(1,32) = 5.5, P = 0.025] and 7-HMG [F(1,24) = 12.92, P = 0.0015], albeit in opposite direc- tions. MG exposure significantly reduced morphine self- administration of 50 μg (P < 0.01), but not 100 μg [Fig. 1d (top panel)]. The decrease in morphine self- administration (50 μg) was not observed on the first day following MG exposure but was significantly de- creased on days two and three Fig. 1c (middle panel)].

Figure 1 Substitution of MG and 7-HMG following morphine self-administration and the impact on subsequent morphine self-administration. (a) Mor- phine was reliably self-administered in a dose-dependent manner (***P < 0.001 saline versus 50 μg/inf and 50 versus 100 μg/inf, **P < 0.01 saline versus 100 μg/inf). (b) MG did not substitute for morphine (100 μg/inf) at any of the doses tested. (c) 7-HMG substituted for morphine (100 μg/inf) in a dose- dependent manner (*P < 0.05 saline versus 5 μg/inf, 10 versus 20 μg/inf, P < 0.01 saline versus 10 μg/inf). (d, e) Comparisons were made between mor- phine self-administration pre-exposure and post-exposure to MG (n = 9), and 7-HMG (n = 7) substitution. (d) Top panel: Comparison of morphine intake pre-MG exposure versus post-MG exposure revealed a significant difference in self-administration (**P < 0.01, pre-MG exposure versus post-MG expo- sure for 50 μg/inf). (d) Middle panel: Assessment of morphine self-administration for 3 days following MG exposure revealed a decrease in intake of 50 μg/ inf morphine compared to pre-MG exposure levels (**P < 0.01 sessions 2 and 3 pre-exposure versus post-exposure to MG). (d) Bottom panel but had no change in morphine self-administration of 100 μg/inf across the 3 days. (e) Top panel, Comparison of morphine intake pre-7-HMG exposure versus post-7-HMG exposure revealed a significant difference in self-administration (**P < 0.01, pre-7-HMG exposure versus post-7-HMG exposure for 50 μg/ inf morphine). (e) Middle panel: Substitution of 7-HMG resulted in a significant increase in morphine self-administration (50 μg/inf) for the 3 days following 7-HMG exposure (**P < 0.01 sessions 1 and 2, *P < 0.05 session 3 pre-exposure versus post-exposure to 7-HMG). (e) Bottom panel whereas self- administration of 100 μg/inf of morphine was not altered by 7-HMG exposure. Data are expressed as mean ± s.e.m.
Intake of 100-μg morphine did not differ from saline before or following exposure to MG [Fig. 1d (bottom panel)].
In contrast, 7-HMG administration significantly increased self-administration of 50-μg morphine (P < 0.01), and there was a trend towards increased intake of 100-μg morphine (P = 0.054) [Fig. 1e (top panel)]. Morphine self-administration (50 μg) was significantly elevated for the three days following 7- HMG exposure by 109, 110 and 95 percent, respectively [Fig. 1e (middle panel)]. Self-administration of 100-μg morphine was also increased for the 3 days following 7-HMG exposure by 53, 75 and 41 percent, respectively [Fig. 1e (bottom panel)]. We also determined whether morphine self-administration was altered by forced absti- nence for an equivalent number of days to the substitution procedure. Morphine intake was not altered by the forced abstinence period [F(1,20) = 8, P = 0.1523], suggesting that the amount of time between morphine self-administration availability did not influence intake (data not shown).
Acquisition of self-administration
Following the assessment of the ability of MG and 7-HMG to substitute for morphine self-administration, we assessed the ability of MG and 7-HMG to engender and maintain responding in a separate cohort of rats. Morphine was readily self-administered at the doses tested [F(2,17) = 24, P < 0.0001]. Self-administration of 50 and 100 μg was greater than saline and the num- ber of infusions obtained for the 50 μg/inf was signifi- cantly greater than 100 μg/inf (Fig. 2a). In contrast, access to MG did not reliably engender or maintain self- administration at any of the doses tested [F(3,31) = 2.255, P = 0.1038] as intake was not signifi- cantly different from vehicle, indicating that MG does not function as a reinforcer in this procedure at the doses tested (Fig. 2b). Similar to morphine, rats self- administered 2.5, 5 and 10 μg/infusion of 7-HMG [F(3,31) = 6.2, P = 0.0024]—the same doses that substituted for morphine self-administration (Fig. 2c). The number of infusions obtained 5 and 10 μg/inf was significantly greater than vehicle (P < 0.05), demon- strating that these doses served as reinforcing stimuli. Intake for 2.5 and 20 μg/inf 7-HMG did not significantly differ from vehicle. Comparison of saline intake for all groups revealed there was no statistically significant difference between the groups [F(2,22) = 2.99, P = 0.0731]. Tukey’s post hoc test revealed there was no statistically significant difference between any of the group pairs.
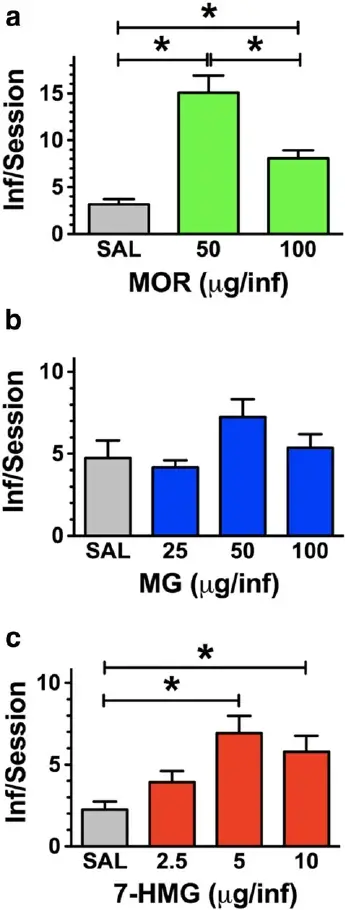
Figure 2 Acquisition of intravenous self-administration with 7- HMG but not MG. (a) Rats acquired morphine self-administration, and intake was dose dependent. (b) Rats did not acquire self-admin- istration of MG at any of the doses tested. (c) However, a separate cohort of rats acquired self-administration of 7-HMG. n = 8 rats. Data are expressed as mean ± s.e.m. *P < 0.05, **P < 0.01, #P < 0.0001
Antagonist effects on 7-HMG and morphine self-administration
The effects of the selective μ opiate receptor antagonist NLXZ and the δ receptor antagonist NTI on morphine (50 μg/inf) and 7-HMG (5 μg/inf) self-administration were examined. The selection was based on doses that maintained the highest intake for morphine and 7-HMG self-administration, respectively. NLXZ pretreatment at- tenuated morphine self-administration [F(2,15) = 76, P < 0.0001] [Fig. 3a (left panel)]. Both 5 and 15 mg/kg NLXZ produced a significant decrease in the number of infusions for morphine (P < 0.05); however, there was no significant difference in the number of infu- sions between the doses. Morphine intake was signifi- cantly decreased the day of NLXZ pretreatment for both doses (P < 0.001) and returned to control levels the following session [Fig. 3a (right panel)]. Pretreatment with NLXZ also significantly reduced 7-HMG self- administration [F(2,22) = 39, P < 0.0001] [Fig. 3b (left panel)]. Both NLXZ doses significantly reduced 7-HMG intake compared to saline (P < 0.0001), although intake following 15 mg/kg was significantly less than following 5 mg/kg (P < 0.05). For both doses, 7-HMG intake was significantly reduced the day of NLXZ pretreatment (P < 0.001) and returned to control levels the following session [Fig. 3b (right panel)]. Comparison of baseline in- take was not significantly different than intake following saline pretreatment for the morphine [t = 2.5, df = 5, P = 0.057] or 7-HMG groups [t = 0.03, df = 7, P = 0.98]; therefore, the number of infusions following saline was used for comparison with NLXZ.

Figure 3 Selective μand δopiate receptor antagonist pretreatment alters 7-HMG self-administration. Effects of NLXZ on morphine (50 μg/inf) and 7-HMG (5 μg/inf) self-administration. Baseline drug intake was not significantly different than intake following saline pretreatment formorphine analysis or 7HMG analysis. (a) Left panel NLXZ significantly reduced the number of morphine infusions compared to saline pretreat-ment (#P<0.0001, saline versus 5 and 15 mg/kg NLXZ). (a) Right panel There was a significant interaction between NLXZ doses and sessionswith infusions on the day of pretreatment significantly less than following saline pretreatment for the 15 mg/kg NLXZ dose (***P<0.001). (b)Left panel NLXZ decreased 7-HMG self-administration, the effect was dose dependent (#P<0.0001, *P<0.05) and (b) right panel There wasa significant interaction between NLXZ doses and sessions with infusions on day of pretreatment significantly less than following saline pretreat-ment for both NLXZ doses. We also examined the effects of NTI on morphine (50 μg/inf) and 7-HMG (5 μg/inf) self-administration. Baselinedrug intake was not significantly different than intake following saline pretreatment for morphine analysis and 7HMG analysis. (c) Left panel NTIdid not significantly alter morphine self-administration at either dose tested. (c) Right panel No significant interaction of NTI dose and session wasobserved. (d) Left panel Pretreatment with NTI decreased 7-HMG self-administration in a dose-dependent manner (**P<0.01, saline versus5.0 mg/kg NTI, 0.5 versus 5.0 mg/kg NTI) and (d) right panel There was a significant interaction between NTI doses and sessions althoughBonferroni’s post hoc analysis did not reveal any significant differences between saline and NTI pretreatment for either day. Data are expressedas mean ± s.e.m.
NTI pretreatment produced differential effects on mor- phine and 7-HMG self-administration. Neither 0.5 nor 5.0 mg/kg NTI significantly altered morphine self- administration [F(2,17) = 0.88, P = 0.434] [Fig. 3c (left panel)]. Morphine intake following either dose of NTI did not differ significantly from saline pretreatment the day of or the day following pretreatment [Fig. 3c (right panel)]. In contrast, 5.0 mg/kg NTI pretreatment signifi- cantly altered 7-HMG self-administration [F(2,23) = 11, P < 0.0007] [Fig. 3d (left panel)]. The effect of NTI on 7-HMG intake was dose dependent as intake following the 5 mg/kg dose was significantly lower than saline (P < 0.05) as well as the 0.5 g/kg dose (P < 0.05). 7-HMG intake was significantly reduced the day of 5.0 mg/kg NTI pretreatment (P < 0.05) and returned to control levels the following session [Fig. 3 (right panel)]. For naltrindole (NTI), comparison of baseline in- take was not significantly different than intake following saline pretreatment for the morphine [t = 0.13, df = 5, P=0.90]or7-HMGgroups[t=0.94,df=7,P=0.38].
DISCUSSION
The present study provides the first characterization of the reinforcing effects of MG and 7-HMG in an animal model of human drug consumption. Using the intrave- nous self-administration procedure, the study demon- strates 7-HMG, but not MG, substitute for morphine self-administration and engender and maintain self- administration in drug-naïve rats. Under both the substitution and acquisition self-administration proce- dures, 7-HMG self-administration exhibited an inverted ‘U’ dose–effect curve, typical of other drugs of abuse under similar experimental conditions, wherein low to moderate doses increase responding and higher doses decrease responding (Wilson, Hitomi, & Schuster 1971; Pickens 1978). In both self-administration procedures, 7-HMG maintained intake above levels observed for saline, indicating the compound serves as a reinforcing stimulus within the range of the doses examined. Previous studies have shown MG induces a conditioned place preference (Sufka et al. 2014; Yusoff et al. 2016; Yusoff et al. 2017) and both MG and 7-HMG generalize to the discriminative stimulus effects of morphine (Harun et al. 2015). Both procedures provide valuable informa- tion about the abuse liability of compounds such as the motivational effects of contextual cues associated with a drug (place conditioning) and whether a compound shares subjective effects with a known drug of abuse (drug discrimination). Self-administration is used to ex- amine the reinforcing effects of drugs, a key feature influencing the potential risk for abuse. With regard to opioids, findings from studies using rat self- administration models are highly consistent with clinical measures of abuse liability and have proven to be a reli- able predictor of abuse liability in humans (O’Connor et al. 2011). While MG shares a similar mechanism of ac- tion with morphine, the present results demonstrate a difference in the reinforcing effects and thus the abuse potential between the two compounds. The discrepancy between the present finding and those of aforementioned place conditioning and drug discrimination studies are intriguing and warrant further assessment of routes of administration as well as non-human primate and hu- man abuse potential studies. The self-administration of 7-HMG along with the shared subjective effects of mor- phine (Harun et al. 2015) indicate that 7-HMG has a sig- nificant potential to be abused. Additional studies are needed to examine the ability of 7-HMG self- administration to induce physical dependence and withdrawal, and the effects of 7-HMG on relapse to morphine, other opiates and other drug classes. The present findings along with the results of additional stud- ies will provide critical information as to whether the compounds warrant control under the Controlled Substances Act.
People also read: Kratom and Future Treatment for The Opioid Addiction and Chronic Pain
In addition to abuse liability assessments, we also explored the impact of exposure to MG and 7-HMG to subsequent morphine intake following completion of the substitution procedure. Exposure to less than 2 mg of MG over a 2-week period (when examining the ability of MG to substitute for morphine) significantly reduced subsequent morphine self-administration up to 3 days following the last administration of MG. These findings raise the possibility that MG may contribute to the reported reduction in opiate intake in dependent individ- uals. Buprenorphine, a μ receptor partial agonist like MG, also reduced morphine (Harrigan & Downs 1981) as well as heroin self-administration (Mello, Bree, & Mendelson 1983) when administered continuously or intravenously twice daily but the effects on intake were acute. The effects of MG on morphine intake in the present study are intriguing but should be interpreted with caution for several reasons including the lack of morphine dose- dependent effects, the absence of a MG dose-effect deter- mination on morphine and other commonly abused opiates, and the need to assess the effect of MG on anima
models of opiate relapse and withdrawal. The finding that MG, in contrast to buprenorphine which has abuse liabil- ity (Jones et al., 2017), does not appear to have abuse li- ability and reduces morphine intake is intriguing and warrants further investigation to determine the efficacy of MG as a potential pharmacotherapy for opiate addic- tion. Comparison of MG with buprenorphine as well as methadone, on the self-administration of morphine as well as other opiates, is needed to determine the thera- peutic potential and potency of MG. The effects of 7- HMG on morphine intake are opposite to those observed with MG. An average total intake of approximately 700 μg of 7-HMG over a 2 to 3-week period (when exam- ining substitution for morphine) induced a significant el- evation in subsequent morphine self-administration over 3 days following the last exposure to the compound, in- dicative of tolerance to morphine. Previous reports indi- cate that kratom users develop tolerance, increasing intake over time (Suwanlert 1975; Hassan et al. 2013). While tolerance to the analgesic effects of 7-HMG has been reported (Matsumoto et al. 2005; Matsumoto et al. 2008), no published studies to date report tolerance or cross-tolerance to morphine (or other opiates) intake. The increase in morphine intake in the present study could reflect a compensatory response to withdrawal from 7-HMG; however, this is unlikely as no overt signs of withdrawal were observed. Additional studies are war- ranted to determine if the observed increase in morphine intake following 7-HMG self-administration is related to a change in the reinforcing effects of morphine, a decrease in the adverse effects or a combination of the two. The demonstration that 7-HMG has significant abuse poten- tial and increases the intake of morphine and possibly other opiates has significant potential clinical relevance.
The present study utilized two variations of the intra- venous self-administration procedure to determine abuse liability of MG and 7-HMG: substitution and acquisition of self-administration. The substitution procedure assessed the ability of MG and 7-HMG to substitute for morphine in rats with a history of morphine self- administration and provided a clinically relevant evalua- tion given the potential for cross-generalization of opi- ates, the use of kratom to reduce intake of and withdrawal from other opiates, and the use of kratom as an affordable substitute for heroin (Boyer, Babu, & Macalino 2007; Boyer et al. 2008; Vicknasingam et al. 2010). The ability of 7-HMG to substitute for morphine in the present study suggests that the reinforcing effects may be mediated by similar neural mechanisms such as μ and δ opiate receptors. The effects of NLXZ on 7-HMG and morphine self-administration may reflect non- specific effects on responding; however, we do not con- sider this a viable interpretation inasmuch as NLXZ doses within this range do not affect responding maintained by saccharin or food (Liu & Jernigan 2011; Peana et al. 2011). The present results confirm and extend previous studies of the effects of NLXZ on opiates including de- creasing heroin self-administration (Negus et al. 1993), decreasing morphine-induced place conditioning in rats (Piepponen et al. 1997) and attenuating the discrimina- tive stimulus effects of morphine (Suzuki et al. 1995). These results indicate that the reinforcing effects of mor- phine 7-HMG are mediated in part by μ opiate receptors.
NTI, the selective δ opiate receptor antagonist, attenu- ated 7-HMG in a dose-dependent manner. The lack of effect of NTI on morphine self-administration is supported by previous studies, which indicates that NTI does not alter morphine place conditioning (Suzuki et al. 1994; Piepponen et al. 1997) or the discriminative stimulus effects of morphine (Stevenson et al. 2000). In contrast, NTI and naltrindole-50 -isothiocyanate have been shown to attenuate the reinforcing effects of heroin (Negus et al. 1993; Martin et al. 2000); however, δ opiate receptors are not considered to directly mediate heroin reinforcement. The reinforcing effects of heroin are medi- ated via μ opiate receptors which bind the major heroin metabolites, morphine and 6-monoacetylmorphine, neither of which bind to the δ receptor with appreciable affinity. The difference in the effects of NTI between the Negus et al. study is likely attributable to the finding that only the 10 and 17 mg/kg doses of NTI were effective in attenuating heroin self-administration—compared with 0.5 and 5 mg/kg in the present study. Given that the 5 mg/kg NTI dose slightly decreased morphine self- administration, higher doses of NTI may have signifi- cantly attenuated intake. The significant attenuation of the reinforcing effects of 7-HMG by NTI reflects involve- ment of δ opiate receptors in the reinforcing effects of this compound. Given that 7-HMG is a weak δ opiate receptor antagonist, the finding that antagonism of the δ receptor partially attenuates the reinforcing effects of this com- pound seems counterintuitive. However, two potential mechanisms may account in part for the finding. Martin et al. suggested that morphine-induced activation of μ opiate receptors in the pallidum stimulates release of met-enkephalin that then binds to δ opiate receptors to exert reinforcing effects (Martin et al. 2000). Because 7-HMG is a partial μ opiate receptor agonist, the com- pound may exert effects similar to morphine via this mechanism. An alternative hypothesis suggests that chronic μ opiate receptor stimulation results in δ opiate receptor recruitment and μ opiate receptor desensitiza- tion. However, the dose-dependent effect of NLXZ on 7-HMG indicates that μ receptors were not de-sensitized. The manner in which δ opiate receptors are involved in 7-HMG reinforcement remains to be determined. None- theless, results from the present study indicate that both μ and δ opiate receptors contribute to the reinforcing effects of 7-HMG. Current pharmacotherapies for opiate addiction including methadone, buprenorphine and nal- oxone do not bind appreciably to δ opiate receptors and therefore may be less effective in treating kratom abuse.
The current results are the first demonstration of self- administration of MG and 7-HMG in the preclinical liter- ature; however, several issues related to study design and methodology deserve to be addressed. First, food was restricted for all subjects in the present study in order to enhance acquisition and maintenance of self- administration in both the substitution and acquisition procedures. Previous studies have demonstrated that food restriction facilitates the acquisition of opiate self- administration and increases intake during the mainte- nance phase as well as enhances drug seeking (Piazza & Le Moal 1998). The absence of MG acquisition or substi- tution under food restriction conditions provides confi- dence that administration of this alkaloid is not reinforcing even under conditions that enhance the prob- ability of intake. Nonetheless, negative results in self- administration procedures are difficult to interpret and may be due to multiple experimental variables that are not optimal for the drug being investigated including, but not limited to, the schedule of reinforcement, re- sponse contingencies, selected doses, rate of infusion, route of administration and drug availability. For exam- ple, the lack of MG self-administration may be attributed in part to the route of administration. Kratom is adminis- tered orally by humans whereas intravenous administra- tion was used in the present study—routes which would yield different rates of onset of drug effects, drug levels, duration of effect and metabolism. The concern is miti- gated to some extent by the finding that 7-HMG, a struc- turally similar compound from the same plant, is readily self-administered intravenously using the same proce- dure. Thus, the relevance of the current findings using the intravenous self-administration is not diminished, al- though future studies need to address the abuse liability of MG and 7-HMG using voluntary oral consumption methods (food and liquid). Another caveat to the present experimental design is the use of one versus two levers in the self-administration procedure. The determination of reinforcement is based on the statistical comparison of in- take maintained by doses of a drug versus a negative con- trol, which is vehicle in the present study. Alternatively, comparisons can be made between the rates of responding on an ‘active’ versus the ‘inactive’ lever. In ei- ther case, drugs that engender and/or maintain responding at levels that exceed responding maintained by the negative control are considered to be self- administered (function as reinforcing stimuli) (O’Connor et al. 2011).
In summary, the present findings from the rodent self- administration model indicate that MG, the main kratom alkaloid, does not have abuse or addiction potential and reduces morphine intake—desired characteristics of candidate pharmacotherapies for opiate addiction and withdrawal. In contrast, 7-HMG should be considered a kratom constituent with high abuse potential that may also increase the intake of other opiates. Although 7-HMG constitutes only 2 percent of the alkaloid content of kratom, purified extracts of 7-HMG are widely available on the internet and are consumed for their euphoric effects. Additional danger is posed by adulteration or the presence of high concentrations of 7-HMG in commercially available kratom products (Lydecker et al. 2016) which may increase the abuse liability.
Source and References: Therapeutic potential and liability of the Mitragyna speciosa (kratom) alkaloids
Comments
Post a Comment